
Table of Standard reduction potentials.pdf - Table of Standard reduction potentials Half reaction + Li + e Li(s) K+ + e K(s) Ca2+ + 2e Ca(s) Na+ + e | Course Hero

Using the standard electrode potentials given in the table, predict if the reaction between the following is possible. Ag^+(aq) and Cu(s)

SOLVED: Table A5.5 Standard Reduction Potentials at 258C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH Fz + 2e 2F 2.87 01 2H,0 + 4e Cu? + Ze Cu Ag + +
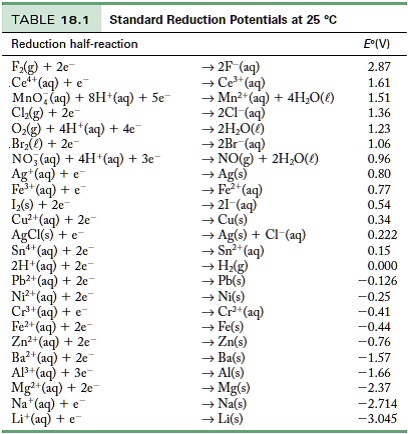
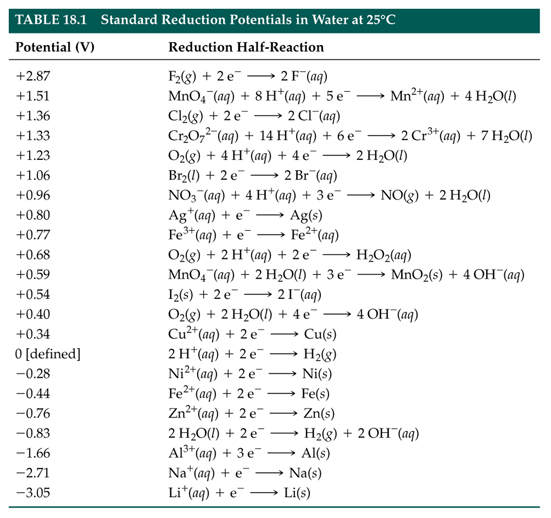
SOLVED: TABLE 18.1 Standard Reduction Potentials at 25 cC Reduction half-reaction Fi(g) (0q MnO (aq 8H+(aq) E'(V) 32F-(aq) 3Ce-(aq) 3Mn?-(aq) 4H,o(e) 2CI-(aq) 72H-O(Q) 2Br (aq) NO(g) ` 2H,O(Q) Ag(s) 3Fe-+(aq) 721-(aq) 3Culs)

Using the standard electrode potentials given in the table, predict if the reaction between the following is possible. Ag^+(aq) and Cu(s)
Half-Reaction +(aq) + 2e- --. Cu(s) Co2+ (aq) + 2e - --. Co(s) PbSO .. (s) + 2e- --. Pb(s) + S~-(aq) Cd2+(aq) + 2e- --. Cd(s) Be
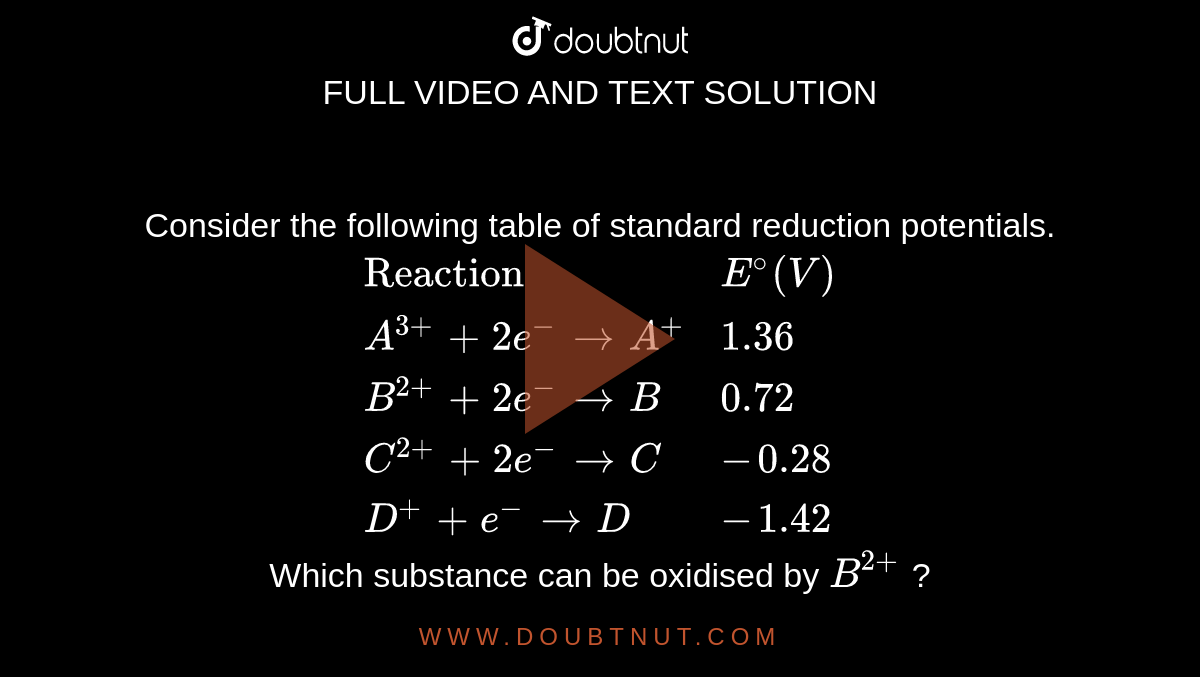
Consider the following table of standard reduction potentials. {:("Reaction",E^@(V)),(A^(3+)+2e^(-) rarrA^+,1.36),(B^(2+)+2e^(-)rarrB,0.72),(C^(2+)+2e^(-)rarrC,-0.28),(D^(+)+e^(-)rarrD,-1.42):} Which substance can be oxidised by B^(2+) ?

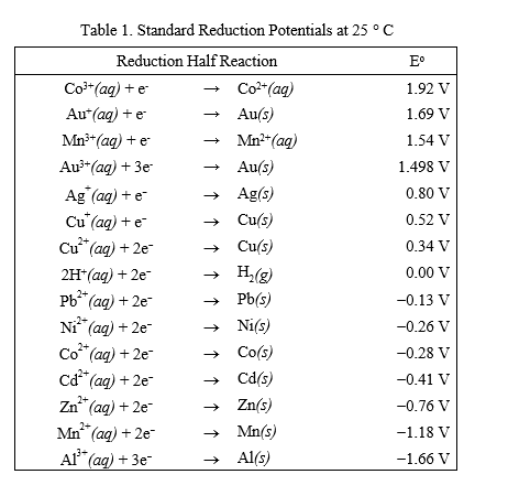
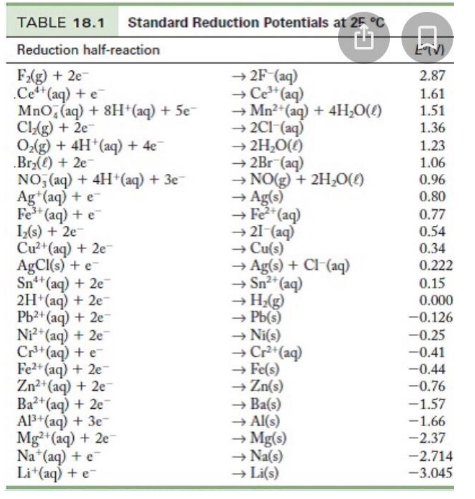
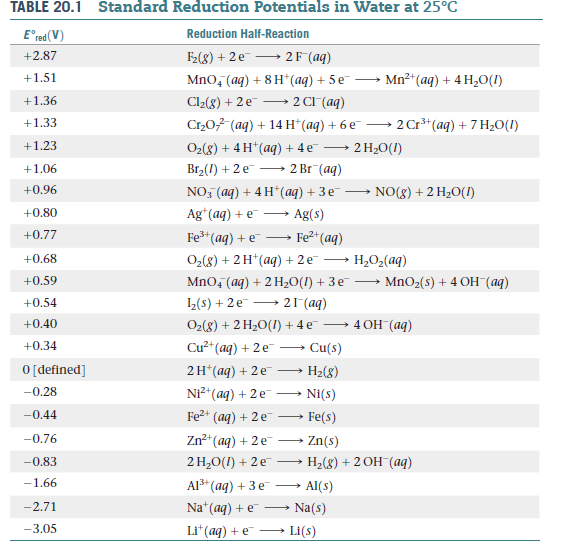

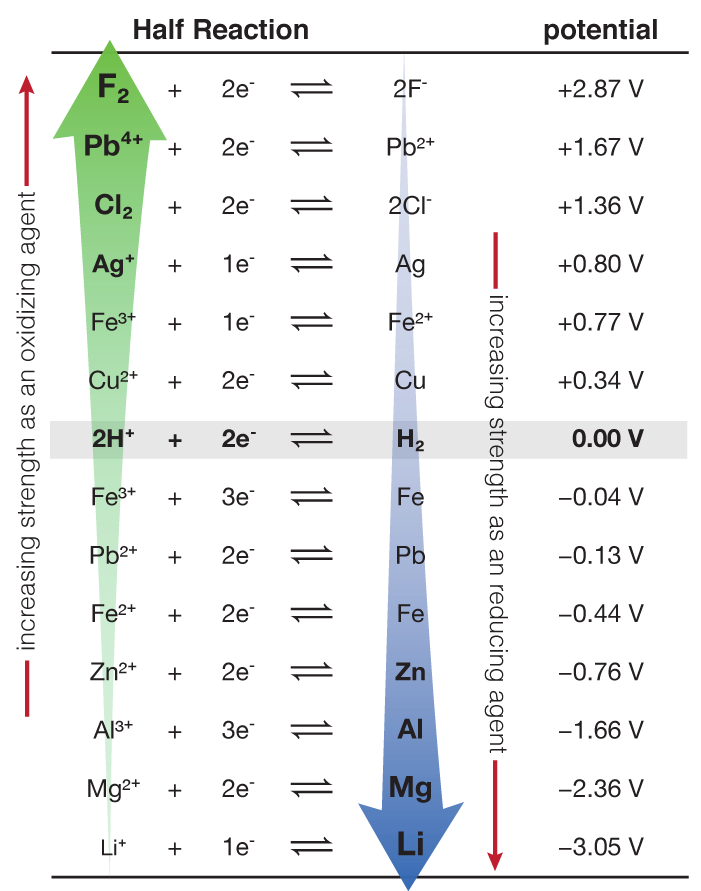

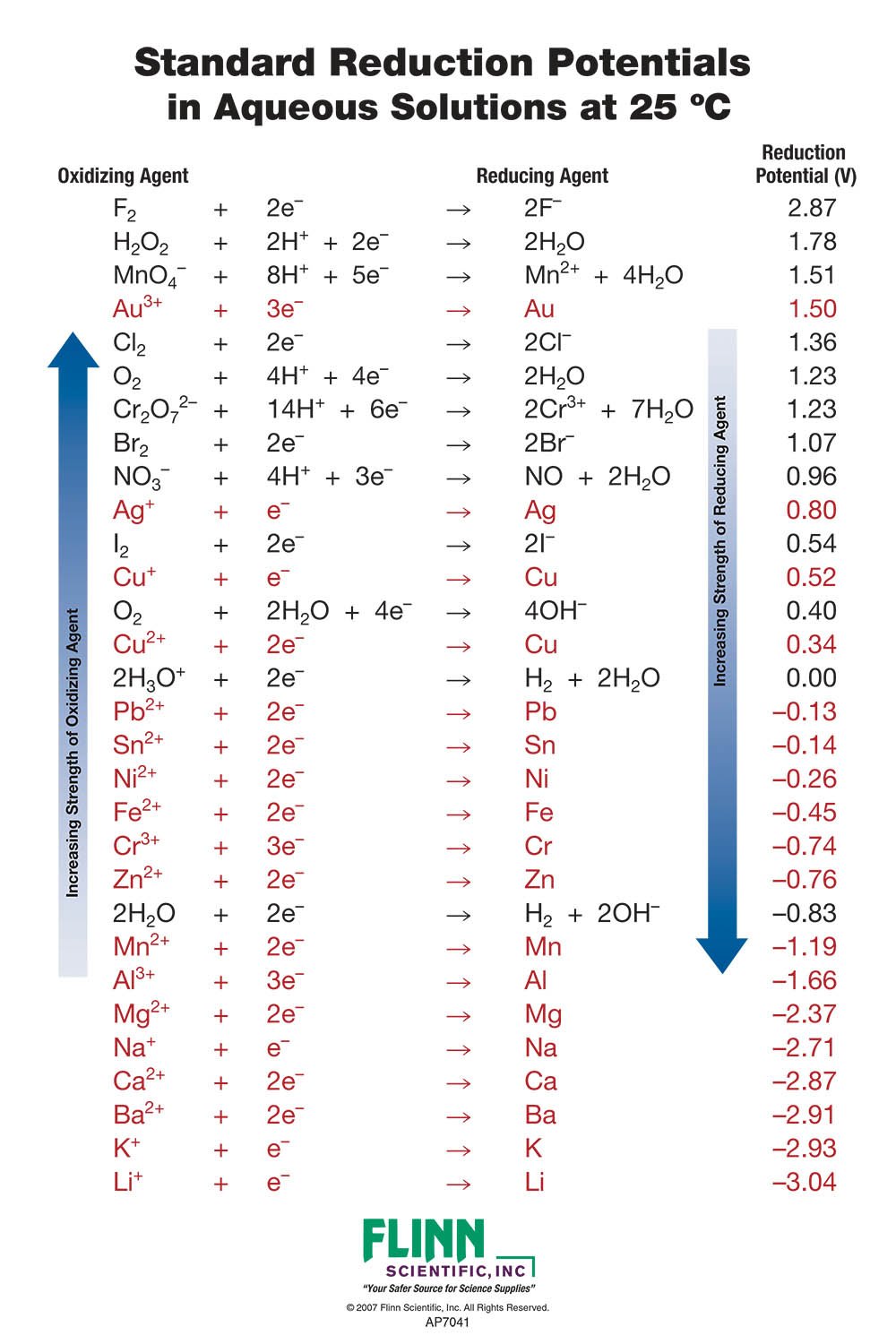


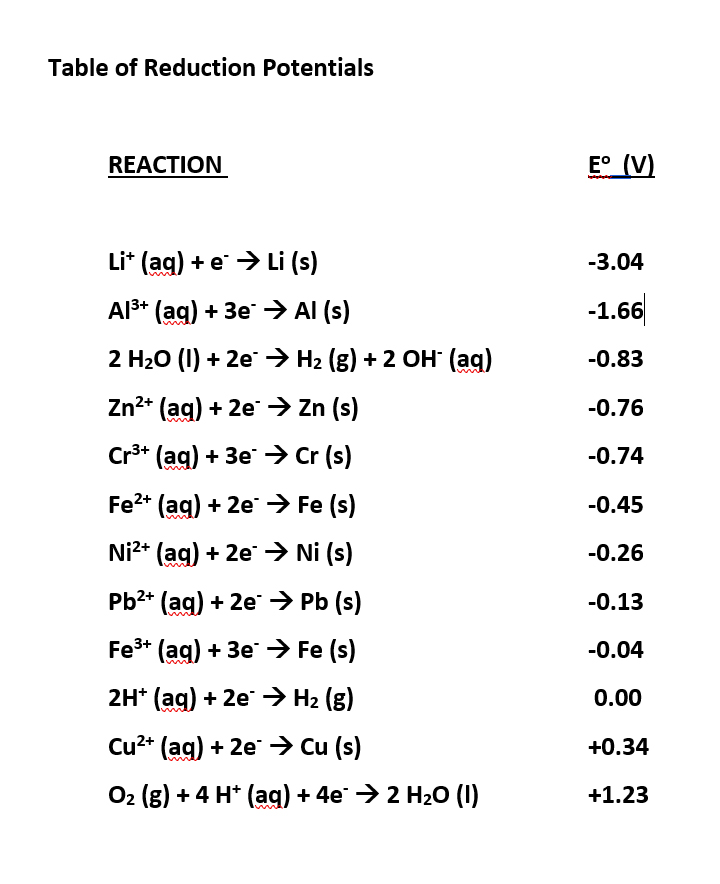

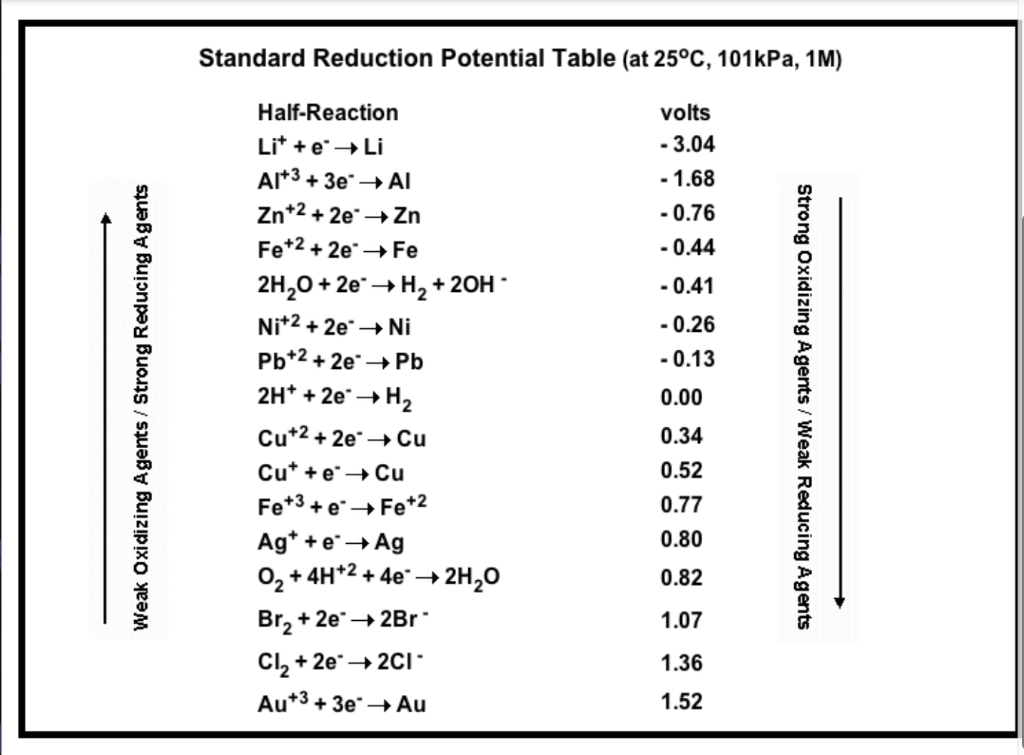

![Standard reduction potentials at 298°K. [24] | Download Table Standard reduction potentials at 298°K. [24] | Download Table](https://www.researchgate.net/publication/316026333/figure/tbl2/AS:650784626708491@1532170554986/Standard-reduction-potentials-at-298K-24.png)